First Cranial Surgery in Latin America Using Immersive Augmented Reality Surgical Navigation
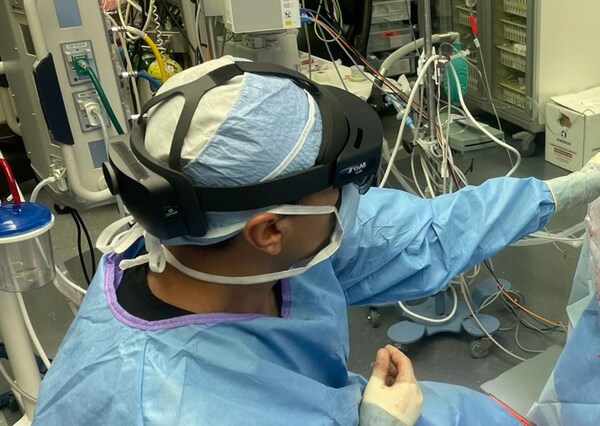
Novarad Corporation used VisAR, their augmented reality surgical navigation system, to biopsy an intraventricular tumor from a patient's brain at MAC CDMX Hospital in Mexico this past week. This surgical procedure marks the first-of-its-kind application of augmented reality surgery in Latin America, showcasing Novarad's commitment to pushing the boundaries of medical science and providing patients with cutting-edge, low-cost treatment options.
In July, surgeons at MAC CDMX Hospital completed a successful spine surgery using VisAR's navigation. Expanding the boundaries of AR navigation, VisAR was utilized in a far more challenging and risky surgery in this case. A team of neurosurgeons, Dr. Alberto Ramirez and Dr. Jose Francisco Cuellar, assisted by Dr. Wendell Gibby of Novarad, utilized VisAR to biopsy a malignant tumor in the 3rd ventricle, deep in the center of the brain.
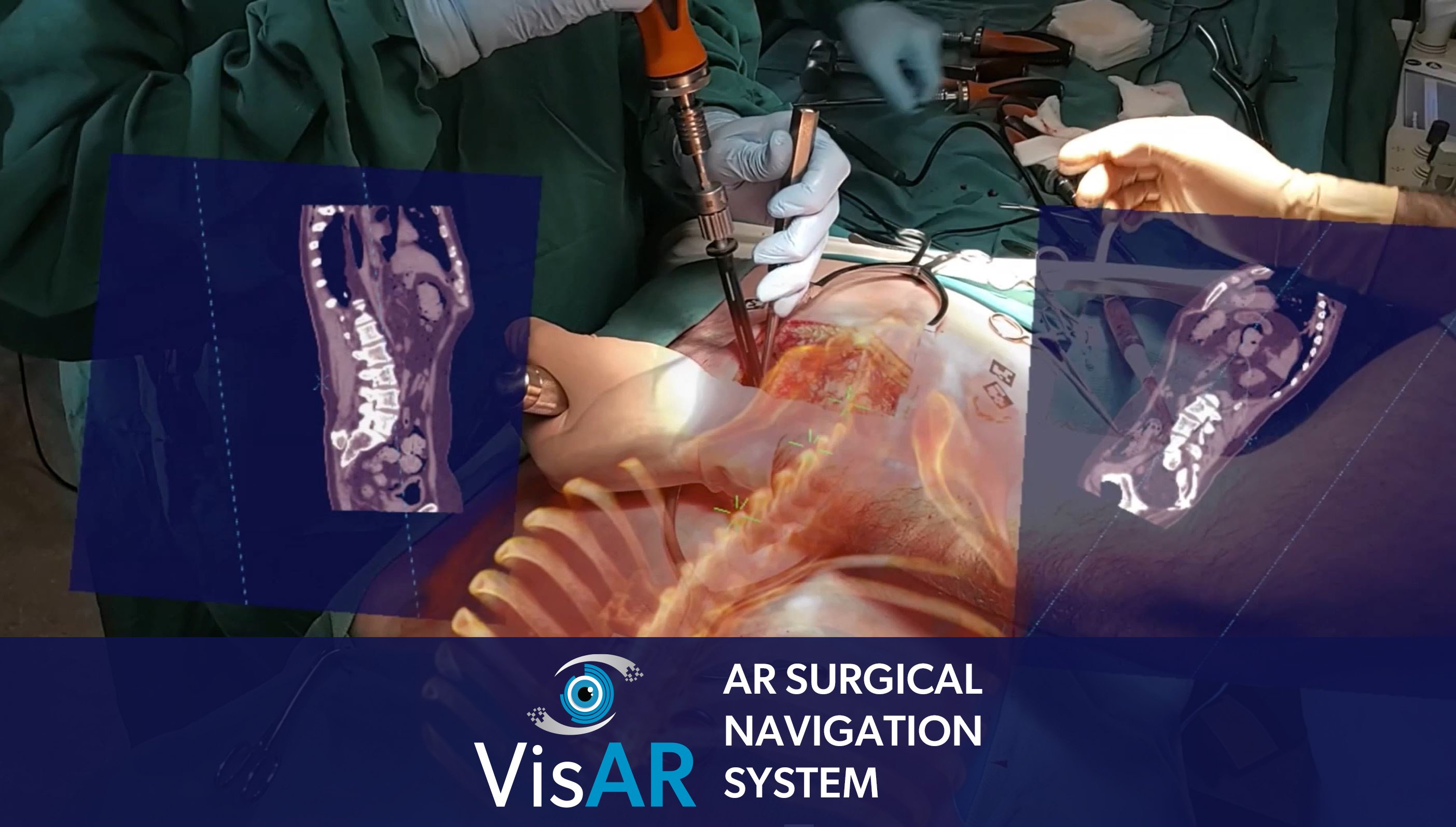
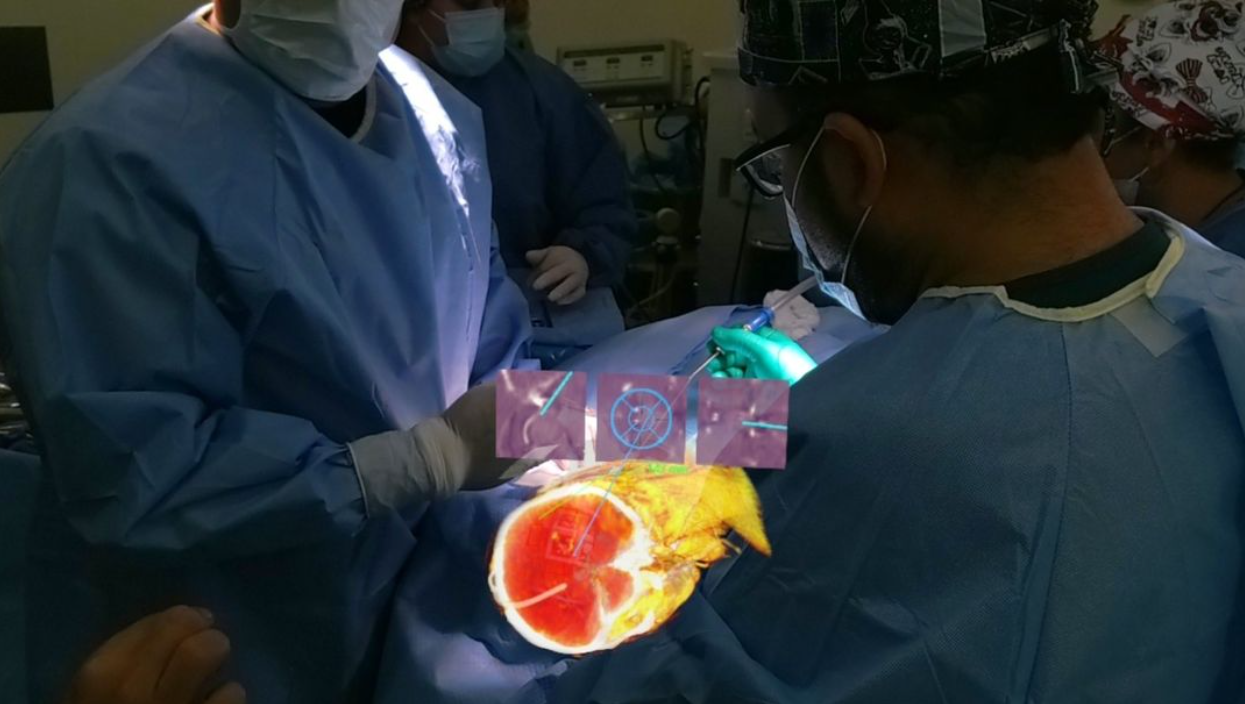
VisAR provides real-time, three-dimensional visualization of the patient's anatomy. It allows precise navigation of surgical instruments with optical tracking via an attached code. This innovative system allows surgeons to superimpose critical information onto the surgical field, providing unparalleled precision and accuracy during delicate surgeries. By enhancing a surgeon's situational awareness, VisAR helps reduce the risk of complications and ensures optimal outcomes.
The precise targeting and biopsy of the intraventricular tumor is as challenging as it gets in neurosurgery. This highlights the potential of VisAR, which uses advanced imaging software paired with an off-the-shelf Microsoft HoloLens to revolutionize delivery of healthcare.
"After performing the first spine cases, we realized that the accuracy and safety with which we can place implants using VisAR is very good," said Dr. Ramirez, one of the surgeons involved in the procedure. "After performing the cranial case, we found we could smoothly and confidently guide our craniotomy margins when entering the anatomical corridor for tumor reception. I think that in some way, the VisAR navigation is essential. Despite a slight increase in surgical time, we see such an improvement during surgery. We have a much better ability to see and understand the brain injury. As I said in the previous spinal surgery, it seems like a revolutionary invention to me."
VisAR began development about 7 years ago. It was the first AR device given FDA clearance for preoperative planning 5 years ago. It has rapidly evolved, achieving stereotactic navigation clearance by the FDA for the spine in 2022. It has been used in eight countries for a wide variety of applications, including spinal implant guidance, plastic surgery, orthopedic trauma and oncology, and interventional radiology.
VisAR is currently available in the United States, Mexico, and Indonesia, with other countries expected to approve the technology in upcoming months. Visit novarad.net/visar for more information or to schedule a demonstration.